 |
|
|
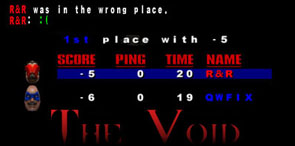
| Topic Starter | Topic: True Story. |
|---|---|
|
ek
Numero Uno  Posts: 3035 |
|
| Top |
|
ek
Numero Uno  Posts: 3035 |
|
||||
| Top |
|
ek
Numero Uno  Posts: 3035 |
|
||||
| Top |
|
ek
Numero Uno  Posts: 3035 |
|
||||
| Top |
|
Scourge
Pestilence  Posts: 15822 |
|
||||
| Top |
|
tnf
guru  Posts: 18068 |
|
||||
| Top |
|
LawL
no homo  Posts: 13721 |
|
||||
| Top |
|
S@M
The Illuminated  Posts: 1623 |
|
||||
| Top |
|
MKJ
Messatsu Ko Jy-ouu  Posts: 44139 |
|
||||
| Top |
|
tnf
guru  Posts: 18068 |
|
||||
| Top |
|
R00k
straight at you  Posts: 27931 |
|
||||
| Top |
|
Pete
Dubuc Coat of Arms  Posts: 904 |
|
||||
| Top |
| Quake3World.com | Forum Index | The Void |
  |